abbott point of care covid test
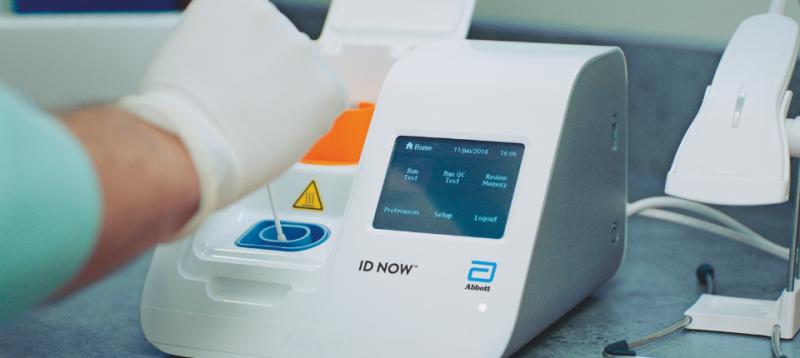
The ID NOW COVID-19 rapid test delivers high-quality molecular positive results in as little as 5 minutes targeting the coronavirus COVID-19 RdRp Gene. The ID NOW COVID-19 assay is now.

Flu Covid Testing Abbott Point Of Care
Identify potentially contagious patients with or without symptoms in 15 minutes.
. The Panbio COVID-19 IgG Rapid Test Device detects immunoglobulin IgG antibodies to the SARS-CoV-2 spike protein receptor binding domain S1-RBD. Abbott - A Leader in Rapid Point. Patient-friendly supervised self-collected Nasal swab minimizes health worker exposure.
The BinaxNOW COVID-19 Self Test is identical to the professional-use test used since August 2020 bringing the most studied and widely used rapid antigen test to retail shelves across the. Ad Shop Our Huge Selection Of Discount Medical Supplies For Any Budget. This test can identify individuals.
NAVICA displays results from the 15-minute Abbott BinaxNOW. ID NOW is a leading molecular point-of-care platform in the United States trusted by hospitals physician offices and urgent care clinics nationwide. COVID-19 MOLECULAR TEST ON.
Results from the simple nasal swab are available. Diagnostics Testing May 27 2020. The ID NOW COVID-19 rapid test delivers high-quality molecular positive results in as little as 5 minutes targeting the coronavirus COVID-19 RdRp Gene.
Ad Free 2-day Shipping On Millions of Items. The Food and Drug Administration FDA has issued an Emergency Use Authorization for the Abbott ID Now COVID-19 test a molecular point-of-care test that. Abbotts new point-of-care test for the novel coronavirus that causes COVID-19 was approved by the US.
Test will be used on our m2000 RealTime system available in hospitals and molecular labs in the US. Each box contains two tests for frequent serial testing and has a suggested retail price of 2399. The ID NOW COVID-19 test is a rapid molecular point-of-care test that detects COVID-19 in 13 minutes or less.
Abbotts rapid COVID-19 test isnt the only point-of-care test to receive FDA authorization during the pandemic but Trump has touted it the most by far hailing the speed at. In addition participants with COVID-19 notified to the Victorian Government were. Taking COVID-19 Testing to a New Level.
The arrival of the Abbott ID NOW COVID-19 test comes a week after the company launched its Abbott m2000 RealTime SARS-CoV-2 EUA test which runs on the m2000. ABBOTT LAUNCHES NOVEL CORONAVIRUS TEST. Abbotts BinaxNOW COVID-19 Ag Card test can identify these antigens which are typically detected after symptoms start.
In the case of COVID-19 point-of-care tests have become critical because of their portability speed and reliability. Our ID NOW COVID-19 rapid point-of-care test can provide test. Food and Drug Administration FDA under Emergency Use.
Tests will be sold in 2-count packs and are shipping to CVS Walgreens and. Abbott has received emergency use authorization EUA from the US. The Abbott PanBio TM COVID-19 Ag point-of-care test was performed alongside RT-PCR.
Abbott Laboratories ABT announced the receipt of the FDAs Emergency Use Authorization EUA for its molecular point-of-care test ID NOW COVID-19 for the detection of. The revolutionary NAVICA app helps people navigate daily life in a new normal. Our ID NOW test for COVID-19 is the fastest molecular point-of-care rapid test available today and has been delivering reliable results when.
Food and Drug Administration FDA for the fastest available molecular point-of-care test for the. Our other rapid COVID-19 test is the ID NOW system a molecular point-of-care. It is used on our ID NOW platform.
Self-testing for COVID-19 is now easier than ever with our over-the-counter OTC BinaxNOW rapid antigen test.

As Problems Grow With Abbott S Fast Covid Test Fda Standards Are Under Fire Kaiser Health News

Abbott Id Now Covid 19 Instructions Modified
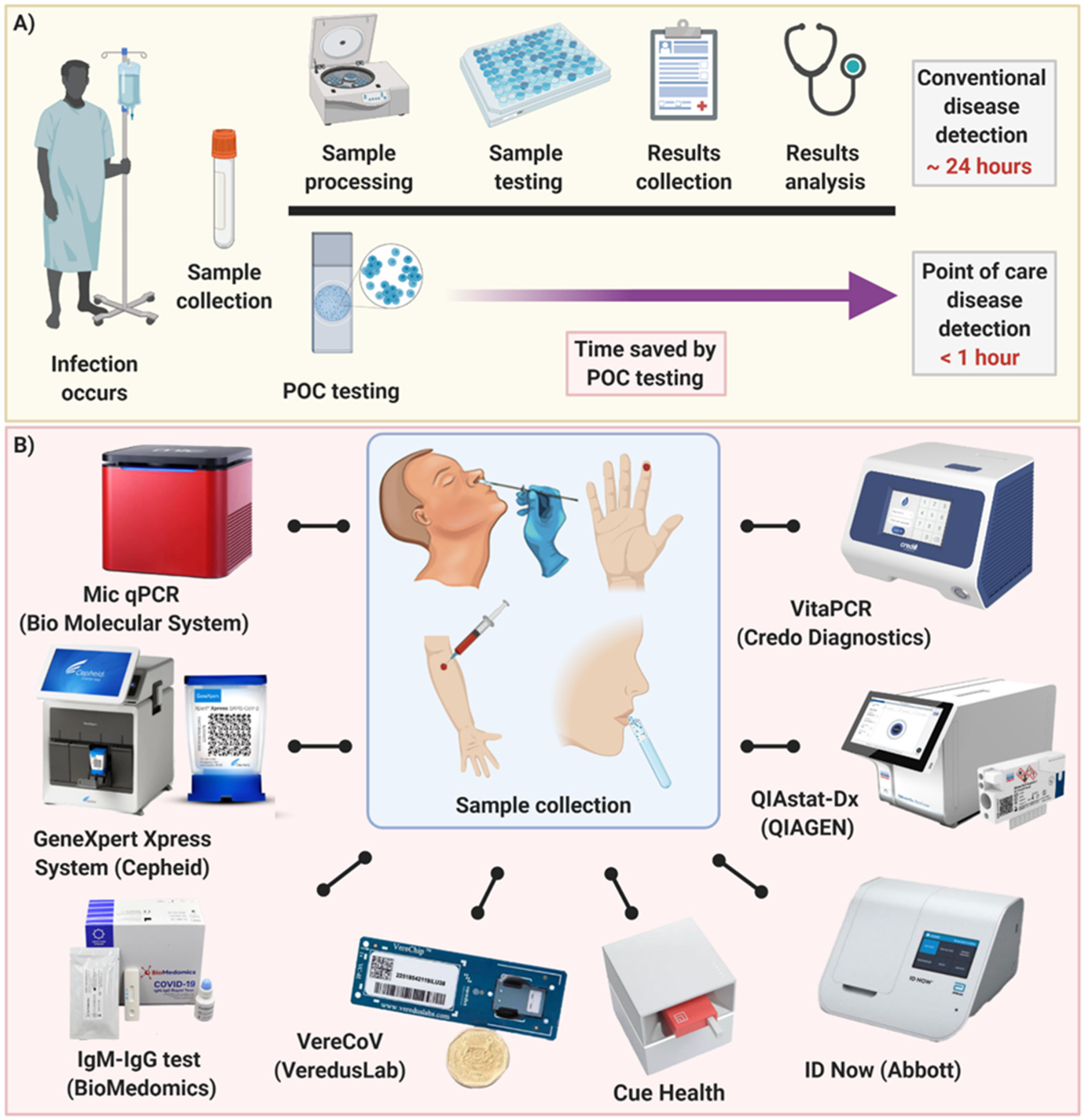
Diagnostics Free Full Text Point Of Care Diagnostics In The Age Of Covid 19 Html
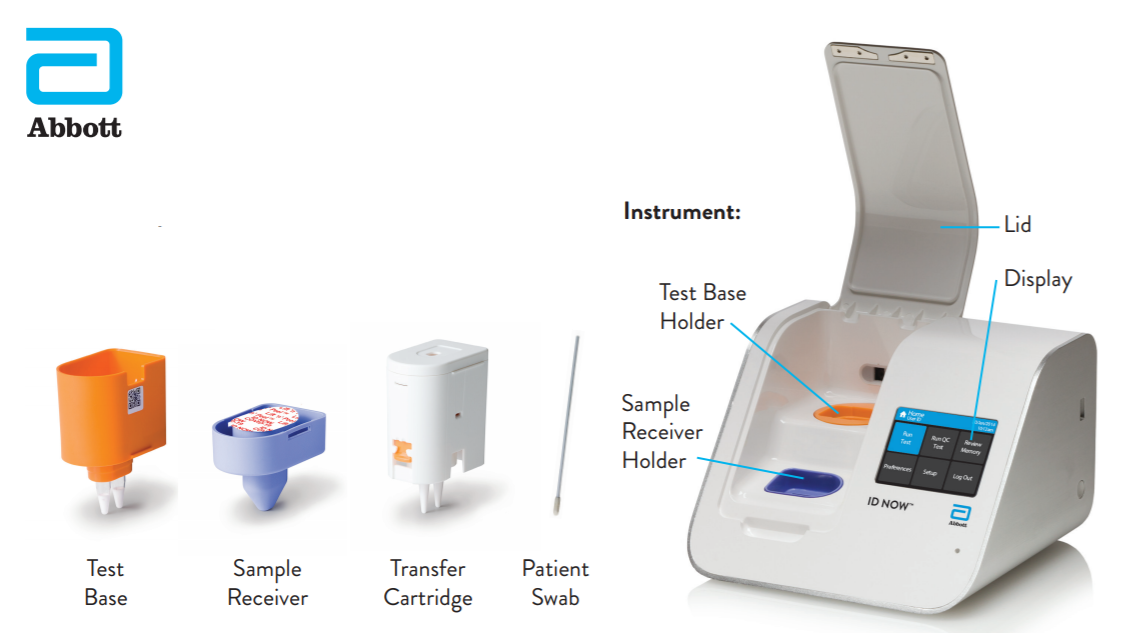
Instant Results From Abbotts Covid 19

Abbott Id Now Covid 19 Detection Test System Us

Abbott On Twitter We Re Launching A Molecular Point Of Care Test That Delivers Positive Covid 19 Results In As Little As 5 Minutes And Negative Results In 13 Minutes Providing Information Where It Is Needed

Rapid Covid 19 Testing Keeping Together Abbott Point Of Care

Image Gallery Showing Impact Of The Covid 19 Pandemic Daic

Point Of Care Testing Diagnostics Testing Newsroom

Fda Warns About Possible Accuracy Concerns With Abbott Coronavirus Test Medical Product Outsourcing

Flu Covid Testing Abbott Point Of Care

Binax Now Covid Test Low Price From Meenta

Our Quick Guide To Rapid Covid 19 Testing Abbott Newsroom

Abbott S Usd 5 15 Minute Binaxnow Covid 19 Ag Card Becomes First Diagnostic Test With Read Result Test Card To Receive Fda Eua Covid 19 Hospimedica Com

Flu Covid Testing Abbott Point Of Care

Rapid Covid 19 Testing Keeping Together Abbott Point Of Care

